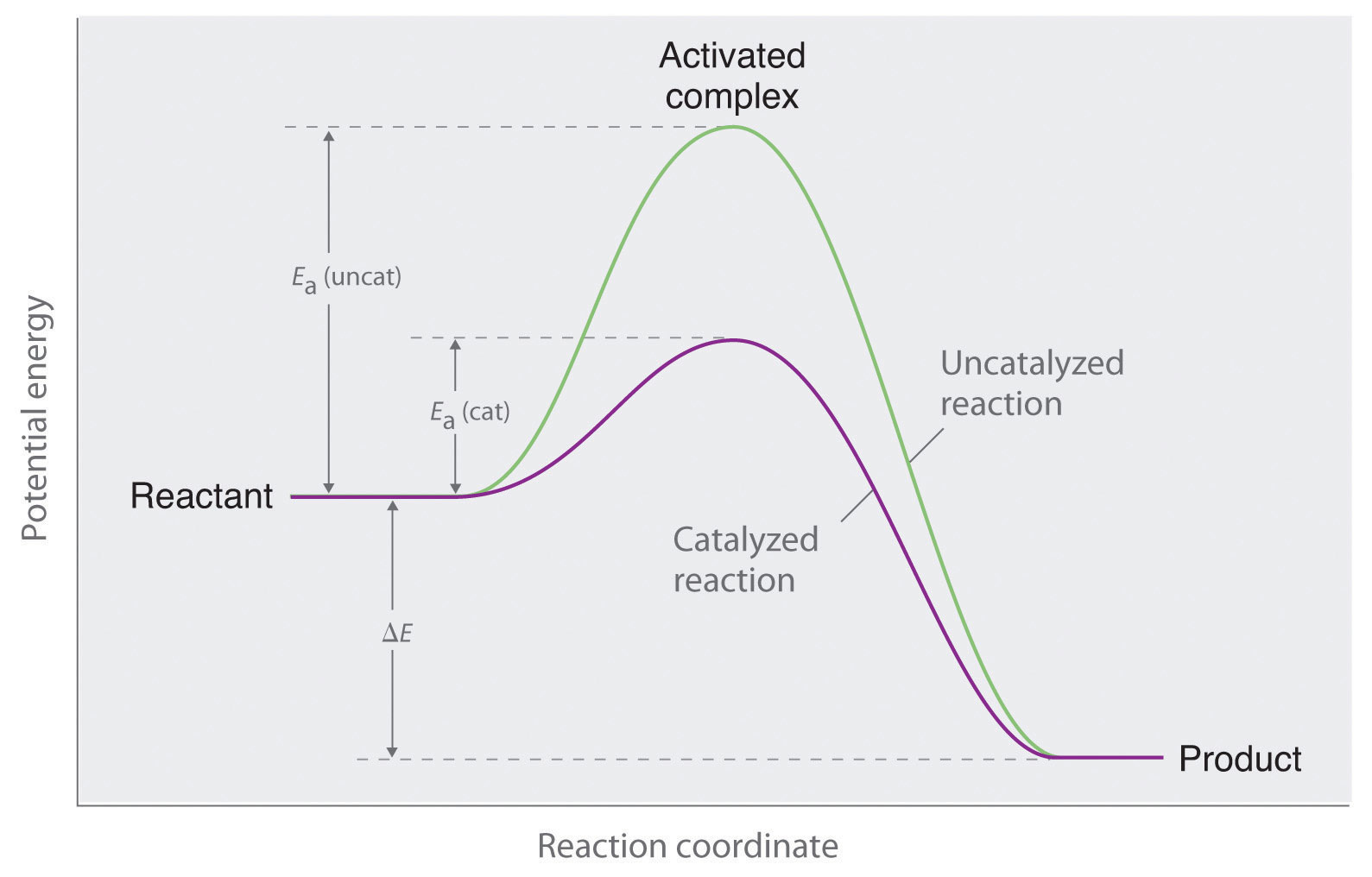
Effect Of Catalyst On Rate Of Reaction Example . A catalyst is a substance which changes the rate of reaction but is unchanged at the end of the reaction. Heterogeneous catalysts are in a different phase from the reactants. Controlling the ways catalysts work is. biological systems use catalysts to increase the rate of the oxidation reaction so that it can occur at a faster rate at lower. catalysts increase the rate of reactions. It assumes familiarity with basic concepts in the. It assumes that you are already familiar with basic ideas about the. By their nature, some reactions occur. this page explains how adding a catalyst affects the rate of a reaction. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. this page titled 6.25: this page describes and explains the way that adding a catalyst affects the rate of a reaction.
from saylordotorg.github.io
this page describes and explains the way that adding a catalyst affects the rate of a reaction. Controlling the ways catalysts work is. catalysts increase the rate of reactions. It assumes familiarity with basic concepts in the. By their nature, some reactions occur. this page titled 6.25: revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. A catalyst is a substance which changes the rate of reaction but is unchanged at the end of the reaction. biological systems use catalysts to increase the rate of the oxidation reaction so that it can occur at a faster rate at lower. It assumes that you are already familiar with basic ideas about the.
Catalysis
Effect Of Catalyst On Rate Of Reaction Example this page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with basic ideas about the. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. Heterogeneous catalysts are in a different phase from the reactants. biological systems use catalysts to increase the rate of the oxidation reaction so that it can occur at a faster rate at lower. this page describes and explains the way that adding a catalyst affects the rate of a reaction. this page titled 6.25: catalysts increase the rate of reactions. Controlling the ways catalysts work is. A catalyst is a substance which changes the rate of reaction but is unchanged at the end of the reaction. this page explains how adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the. By their nature, some reactions occur. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate.
From www.youtube.com
6.2.6 / 6.2.7 Describe the effect of a catalyst on a chemical reaction Effect Of Catalyst On Rate Of Reaction Example It assumes that you are already familiar with basic ideas about the. biological systems use catalysts to increase the rate of the oxidation reaction so that it can occur at a faster rate at lower. Controlling the ways catalysts work is. catalysts increase the rate of reactions. this page titled 6.25: A catalyst is a substance which. Effect Of Catalyst On Rate Of Reaction Example.
From courses.lumenlearning.com
Catalysis General Chemistry Effect Of Catalyst On Rate Of Reaction Example By their nature, some reactions occur. biological systems use catalysts to increase the rate of the oxidation reaction so that it can occur at a faster rate at lower. Controlling the ways catalysts work is. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. catalysts increase the rate of reactions. It assumes familiarity. Effect Of Catalyst On Rate Of Reaction Example.
From www.nagwa.com
Question Video Understanding How Catalysts Affect the Rates and the Effect Of Catalyst On Rate Of Reaction Example revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. this page describes and explains the way that adding a catalyst affects the rate of a reaction. this page explains how adding a catalyst affects the rate of a reaction. A catalyst is a substance which changes. Effect Of Catalyst On Rate Of Reaction Example.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Effect Of Catalyst On Rate Of Reaction Example biological systems use catalysts to increase the rate of the oxidation reaction so that it can occur at a faster rate at lower. Heterogeneous catalysts are in a different phase from the reactants. By their nature, some reactions occur. It assumes familiarity with basic concepts in the. revise how to measure rates of reaction and how to impact. Effect Of Catalyst On Rate Of Reaction Example.
From www.youtube.com
Effect of Catalyst on Rate of Reaction Chemical Chemistry Effect Of Catalyst On Rate Of Reaction Example A catalyst is a substance which changes the rate of reaction but is unchanged at the end of the reaction. By their nature, some reactions occur. biological systems use catalysts to increase the rate of the oxidation reaction so that it can occur at a faster rate at lower. It assumes familiarity with basic concepts in the. revise. Effect Of Catalyst On Rate Of Reaction Example.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Effect Of Catalyst On Rate Of Reaction Example A catalyst is a substance which changes the rate of reaction but is unchanged at the end of the reaction. Heterogeneous catalysts are in a different phase from the reactants. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. catalysts increase the rate of reactions. this page titled 6.25: biological systems use. Effect Of Catalyst On Rate Of Reaction Example.
From www.expii.com
Factors Affecting Reaction Rate — Overview & Examples Expii Effect Of Catalyst On Rate Of Reaction Example this page explains how adding a catalyst affects the rate of a reaction. this page titled 6.25: this page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst is a substance which changes the rate of reaction but is unchanged at the end of the reaction. revise how. Effect Of Catalyst On Rate Of Reaction Example.
From www.w3schools.blog
Factors affecting the rate of a reaction Temperature W3schools Effect Of Catalyst On Rate Of Reaction Example this page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst is a substance which changes the rate of reaction but is unchanged at the end of the reaction. Controlling the ways catalysts work is. It assumes that you are already familiar with basic ideas about the. this page explains. Effect Of Catalyst On Rate Of Reaction Example.
From www.youtube.com
How Catalysts Affect Rate Of Reaction GCSE Chemistry Effect Of Catalyst On Rate Of Reaction Example revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. A catalyst is a substance which changes the rate of reaction but is unchanged at the end of the reaction. Controlling the ways catalysts work is. catalysts increase the rate of reactions. this page describes and explains. Effect Of Catalyst On Rate Of Reaction Example.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science Effect Of Catalyst On Rate Of Reaction Example It assumes that you are already familiar with basic ideas about the. this page describes and explains the way that adding a catalyst affects the rate of a reaction. Controlling the ways catalysts work is. this page explains how adding a catalyst affects the rate of a reaction. A catalyst is a substance which changes the rate of. Effect Of Catalyst On Rate Of Reaction Example.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Effect Of Catalyst On Rate Of Reaction Example Controlling the ways catalysts work is. It assumes familiarity with basic concepts in the. By their nature, some reactions occur. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. biological systems use catalysts to increase the rate of the oxidation reaction so that it can occur at. Effect Of Catalyst On Rate Of Reaction Example.
From www.hanlin.com
Edexcel IGCSE Chemistry 复习笔记 3.2.6 Practical Effect of Catalysts on Effect Of Catalyst On Rate Of Reaction Example Heterogeneous catalysts are in a different phase from the reactants. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. It assumes familiarity with basic concepts in the. biological systems use catalysts to increase the rate of the oxidation reaction so that it can occur at a faster rate at lower. catalysts increase the. Effect Of Catalyst On Rate Of Reaction Example.
From byjus.com
How does a catalyst increase the rate of a reaction? Effect Of Catalyst On Rate Of Reaction Example It assumes that you are already familiar with basic ideas about the. biological systems use catalysts to increase the rate of the oxidation reaction so that it can occur at a faster rate at lower. describe how temperatures, concentration of reactant, and a catalyst affect the reaction rate. this page explains how adding a catalyst affects the. Effect Of Catalyst On Rate Of Reaction Example.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Effect Of Catalyst On Rate Of Reaction Example revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. A catalyst is a substance which changes the rate of reaction but is unchanged at the end of the reaction. this page titled 6.25: It assumes familiarity with basic concepts in the. this page describes and explains. Effect Of Catalyst On Rate Of Reaction Example.
From shapeguidance1.gitlab.io
Outrageous Does A Catalyst Increase The Rate Of Reaction Year 12 Effect Of Catalyst On Rate Of Reaction Example Heterogeneous catalysts are in a different phase from the reactants. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. biological systems use catalysts to increase the rate of the oxidation reaction so that it can occur at a faster rate at lower. this page describes and. Effect Of Catalyst On Rate Of Reaction Example.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry Effect Of Catalyst On Rate Of Reaction Example A catalyst is a substance which changes the rate of reaction but is unchanged at the end of the reaction. By their nature, some reactions occur. It assumes that you are already familiar with basic ideas about the. this page explains how adding a catalyst affects the rate of a reaction. describe how temperatures, concentration of reactant, and. Effect Of Catalyst On Rate Of Reaction Example.
From www.expii.com
Factors Affecting Reaction Rate — Overview & Examples Expii Effect Of Catalyst On Rate Of Reaction Example this page titled 6.25: catalysts increase the rate of reactions. Heterogeneous catalysts are in a different phase from the reactants. this page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst is a substance which changes the rate of reaction but is unchanged at the end of the reaction.. Effect Of Catalyst On Rate Of Reaction Example.
From deborahjbivonaxo.blob.core.windows.net
Catalysts That Change The Rates Of Biochemical Reactions Effect Of Catalyst On Rate Of Reaction Example By their nature, some reactions occur. revise how to measure rates of reaction and how to impact the rate by changing the temperature, concentration and surface. catalysts increase the rate of reactions. this page titled 6.25: It assumes familiarity with basic concepts in the. describe how temperatures, concentration of reactant, and a catalyst affect the reaction. Effect Of Catalyst On Rate Of Reaction Example.